- info@teccro.org
- Mumbai, India
- Contact Us
14May
Patient-Centric Trial Design: What It Means and Why It Matters
by admin, 0 Comments
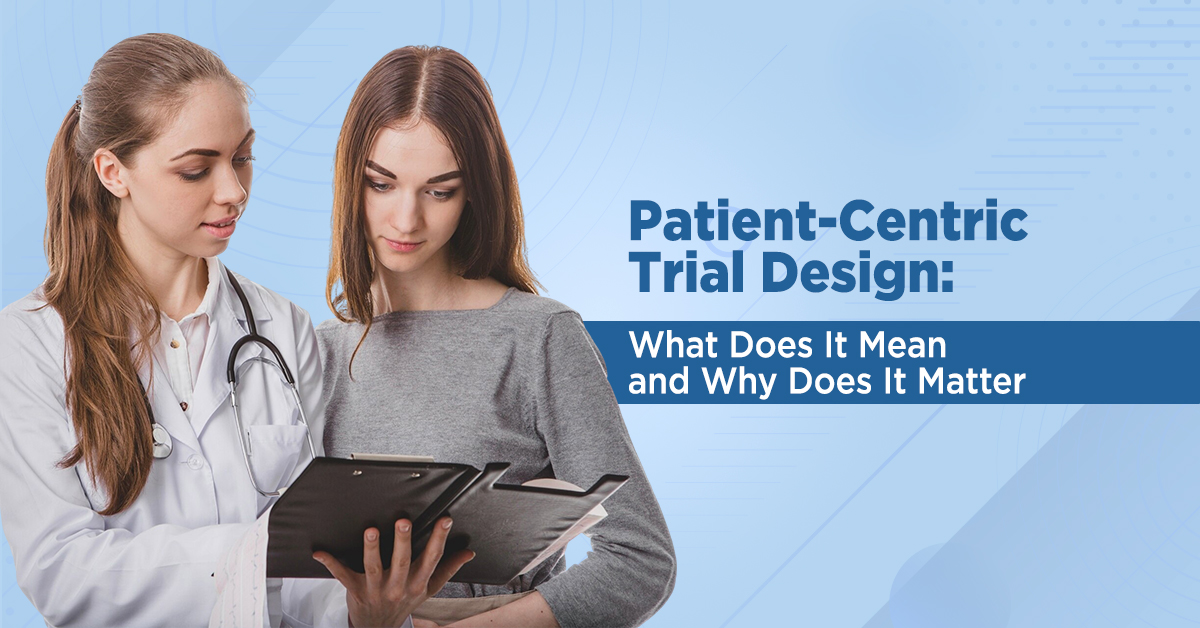
In traditional clinical research, patients have often been treated as passive participants -recipients of treatments who report symptoms and follow protocols. But the landscape is changing. The future of clinical trials, especially in dermatology and cosmetology, is patient-driven.
At TECCRO (The Esthetic Clinics Clinical Research Organization), we champion a new paradigm: one where patients are not only involved but are central to how studies are designed, conducted, and interpreted. Because when patients are heard, science moves forward with purpose.
What Is Patient-Centric Trial Design?
A patient-centric trial design places the patient’s needs, preferences, and lived experiences at the heart of clinical research. It’s not just about measuring a treatment’s effectiveness – it’s about understanding how patients feel, function, and recover throughout their journey.
Key principles include:
- Involving patients in protocol development and feedback mechanisms
- Reducing participant burden (e.g., fewer visits, user-friendly consent materials)
- Promoting transparency in trial outcomes and communication
- Prioritizing quality of life (QoL) over purely laboratory-based metrics
- Treating patients as active collaborators, not passive subjects
This approach transforms trials from something done to patients into something done with them.
Why Patient-Centricity Matters in Cosmetology Research
Cosmetic and aesthetic treatments are deeply personal. Whether addressing hair loss, pigmentation, or signs of aging, these interventions aim to restore not just appearance – but confidence, self-esteem, and emotional well-being.
Why this matters in research:
- Improves recruitment and retention: Studies designed with patient convenience in mind are more likely to attract and retain participants.
- Enhances compliance: Patients who understand and align with the purpose of a study are more likely to adhere to its protocols.
- Captures real-world outcomes: Patient-reported outcomes (PROs) offer valuable insights that go beyond clinical metrics – capturing emotional and social dimensions of treatment success.
- Builds lasting trust: Open communication and involvement foster trust in research institutions and in the safety of aesthetic innovations.
TECCRO’s Approach: Trials Designed with the Patient in Mind
At TECCRO, patient-first thinking is woven into every phase of our clinical studies. Whether it’s a trial for injectables, lasers, topicals, or emerging therapies, we balance scientific precision with patient experience.
- Simplified Consent & Education
We use visual aids, regional languages, and interactive Q&A sessions to ensure participants fully understand their roles and rights in the trial.
- Comfort-Oriented Protocols
Minimizing invasive procedures, offering digital follow-ups, and reducing travel burdens are standard practices in our trials.
- Patient-Reported Outcome Measures (PROMs)
We evaluate metrics like confidence, social engagement, and satisfaction with appearance – because in aesthetic medicine, these are just as meaningful as technical scores.
- Ethics Committee Oversight
All studies are reviewed by our DCGI- and CDSCO-recognized Institutional Ethics Committee (IEC – The Esthetic Clinics), ensuring patient rights, safety, and voices are prioritized.
Looking Ahead: The Patient as Partner
When patients are treated as partners in research, the entire ecosystem benefits – richer data, faster innovation, and more relevant results. In aesthetic medicine, where treatments aim to enhance well-being and self-image, this approach isn’t optional – it’s essential.
At TECCRO, we don’t just conduct clinical trials.
We co-create them – with patients, for patients.
Related posts:
 The Role of CROs in Accelerating Drug Development
The Role of CROs in Accelerating Drug Development
 The Vital Role of Ethics Committees in Ensuring Ethical Clinical Research
The Vital Role of Ethics Committees in Ensuring Ethical Clinical Research
 How Conventional Treatments Spark New Innovations in Medical Healthcare
How Conventional Treatments Spark New Innovations in Medical Healthcare
 The Role of Clinical Research in Cosmetology: Ensuring Safety, Efficacy, and Innovation
The Role of Clinical Research in Cosmetology: Ensuring Safety, Efficacy, and Innovation
 The Impact of Treatment Costs on Surgical Decision-Making
The Impact of Treatment Costs on Surgical Decision-Making
 The Crucial Role of Precision in Delivering Optimal Patient Care
The Crucial Role of Precision in Delivering Optimal Patient Care
 Accelerating Medical Innovation: TECCRO’s Expertise in Patent Filing for Breakthrough Solutions
Accelerating Medical Innovation: TECCRO’s Expertise in Patent Filing for Breakthrough Solutions
 Revolutionizing Beauty: How Advanced Cosmetic Treatments Are Changing Lives
Revolutionizing Beauty: How Advanced Cosmetic Treatments Are Changing Lives
Search
Recent Posts
- Challenges in Placebo Design for Aesthetic and Hair Loss Studies
- Long-Term Safety Outcomes in Fillers: What Do Clinical Studies Show?
- Cultural Perceptions of Beauty and Their Impact on Trial Recruitment
- The Rise of Investigator-Initiated Trials in Aesthetic Dermatology
- Recruitment Challenges in Aesthetic Clinical Trials and How to Overcome Them
About TECCRO

We at The Esthetic Clinics Clinical Research Organization (TECCRO) believe that Clinical Research Organizations (CRO) necessarily need to have the best clinicians so that the pharmaceutical sponsors can be guided strongly on what would be the best way to carry their study protocols forwards, to achieve their means. In this sense, our clinical team provides a clear & immense differentiator and that is we The Esthetic Clinics Clinical Research Organization (TECCRO) is consistently rated amongst the Best Clinical Research Organizations in India by industry and pharmaceutical companies. Read more..
